iodine orbital diagram|Iodine orbital diagram : Tuguegarao I (Iodine) is an element with position number 53 in the periodic table. Located in the V period. Melting point: 113.5 ℃. Density: 4.94 g/cm 3 . Watch Hot Pinoy Blowjob gay porn videos for free, here on Pornhub.com. Discover the growing collection of high quality Most Relevant gay XXX movies and clips. No other sex tube is more popular and features more Hot Pinoy Blowjob gay scenes than Pornhub! Browse through our impressive selection of porn videos in HD quality on any device you .
PH0 · Iodine orbital diagram
PH1 · Iodine Electron Configuration: Everything You Need to Know
PH2 · Iodine Electron Configuration (I) with Orbital Diagram
PH3 · Iodine
PH4 · How to Write the Orbital Diagram for Iodine (I)?
PH5 · How to Write the Orbital Diagram for Iodine (I)?
PH6 · Exploring the Intricate I2 Molecular Orbital Diagram: A
PH7 · Electron configuration for Iodine (element 53). Orbital diagram
PH8 · Complete Electron Configuration for Iodine (I, I– ion)
PH9 · 5.1: Electron Configurations
PH10 · 2.4 Electron Configurations
Irish Lotto Statistics. See the latest Irish Lotto statistics for all draws from Saturday 5th September 2015 up to and including Saturday 31st August 2024. These stats are updated after every draw and display lots of useful information, including how often each number has been selected in the winning line, the most commonly selected numbers .
iodine orbital diagram*******The iodine orbital diagram is a graphical representation of the electron configuration of the iodine atom. This diagram shows how the electrons in the iodine atom are arranged in different orbitals.
When depicting the iodine orbital diagram, start by determining the number of electrons from the periodic table. Use the electron configuration for reference and follow the three fundamental . Iodine is the least abundant of the stable halogens and also the sixty-first most abundant element. Today we are going to give you all the information related to the electron configuration of the Iodine.I (Iodine) is an element with position number 53 in the periodic table. Located in the V period. Melting point: 113.5 ℃. Density: 4.94 g/cm 3 .The I2 molecular orbital diagram provides a visual representation of the electronic structure of the iodine molecule and helps in understanding its properties, such as .
An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons.Element Iodine (I), Group 17, Atomic Number 53, p-block, Mass 126.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We start with a single hydrogen atom (atomic .
iodine orbital diagram Iodine electron configuration diagram. The electronic configuration of [I] is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4d10 5s2 5p5. The diagram drawn is as follows, where- The .Iodine (I) has an atomic mass of 53. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ChemicalAid. . Orbital Diagram. Nuclear. Radioactive: No: Isotopes. Symbol Mass Number Relative Atomic Mass Isotopic Composition; 108 I: 108: 107.94348(39)# 109 I: 109: 108.93815(11) 110 I: 110: .
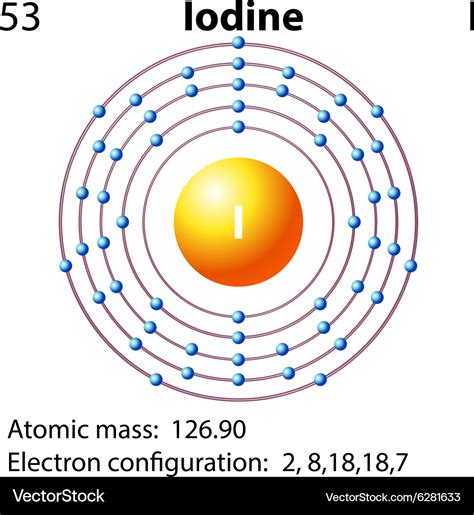
Iodine atoms have 53 electrons and the shell structure is 2.8.18.18.7. The ground state electron configuration of ground state gaseous neutral iodine is [Kr].4d 10.5s 2.5p 5 and the term symbol is 2 .
The second excited state of Iodine Ground state Iodine orbital diagram. The ground state electronic configuration of I is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5 and the orbital diagram is drawn using the following steps. At first, the orbitals are arranged in increasing order of energy. We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We start with a single .
Iodine orbital diagram Iodine Orbital Diagram. Iodine configuration electronic atoms structure webelements properties schematic Iodine structure atomic model bohr element symbol data. 29 Jun 2024. Solved which is the correct hybrid orbital diagram Orbital diagram hybrid correct iodine hybridization representing which transcribed text show . When a figure is built, its bounding box is updated as it is built. This bounding box is a node named current bounding box.. So I traced its left side by shifting it to the left at the very end of the code.
Iodine Orbital Diagram. Configuration iodine electronic ion Orbital diagram hybrid correct iodine hybridization representing which transcribed text show. 08 Aug 2023. A step-by-step description of how to write the electron configuration Aufbau principle: electrons are placed into the orbitals of lowest elements periodic table » iodine .You can probably draw the Lewis structure and molecular orbital diagram of \(\ce{I2}\). . In other words, the iodine atoms move in space with respect to each other, which effects the energy of the initial state. Furthermore, the electron excitation alters the bonding in the molecule, effecting vibrational states.iodine orbital diagram Iodine orbital diagram The 1s orbital and 2s orbital both have the characteristics of an s orbital (radial nodes, spherical volume probabilities, can only hold two electrons, etc.) but, as they are found in different energy levels, they occupy different spaces around the nucleus. Each orbital can be represented by specific blocks on the periodic table.Figure 2.5.8 Orbital Energy Level Diagram for the Hydrogen Atom Each box corresponds to one orbital. Note that the difference in energy between orbitals decreases rapidly with increasing values of n. The energies of the orbitals in any species with only one electron can be calculated by a minor variation of Bohr’s equation . Orbital diagram of elements; Atomic radii of elements; Ionization energies of elements; Electron configuration of elements; Protons, neutrons, electrons of elements . Electron configuration of .

The iodine 5p z orbital is hybridized with the hydrogen 1s orbital and forms an antibonding σ * orbital responsible for transitions E in the absorption spectrum (see Figs. 4 and 6).Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We start with a single hydrogen atom (atomic number 1), which consists .
Iodine (I). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of iodine-127 (atomic number: 53), the most common isotope of this element. The nucleus consists of 53 protons (red) and 74 neutrons (orange). 53 electrons (white) successively occupy available electron shells (rings).
Iodine Facts, Symbol, Discovery, Properties, Uses. Check Details. Electronic Configuration - Iodine ion - YouTube. Check Details. 10 Interesting Iodine Facts | My Interesting Facts. Check Details. Solved Which is the correct hybrid orbital diagram | Chegg.com. Check Details. Symbol and electron diagram for iodine Royalty Free VectorFilling Orbital: 5p 5; Number of Electrons (with no charge): 53; Number of Neutrons (most common/stable nuclide): 74; Number of Protons: 53; Oxidation States: ±1,5,7; . Iodine - I (EnvironmentalChemistry.com)- Comprehensive information for the element Iodine - I is provided by this page including scores of properties, element names in . Let, n = 1 for K orbit. So, the maximum electron holding capacity in the K orbit is 2n 2 = 2 × 1 2 = 2 electrons. n = 2, for L orbit. The maximum electron holding capacity in the L orbit is 2n 2 = 2 × 2 2 = 8 electrons. n=3 for M orbit. The maximum electron holding capacity in the M orbit is 2n 2 = 2 × 3 2 = 18 electrons. n=4 for N orbit.Figure \(\PageIndex{3}\): This is the valence MO diagram of HF. The H1s orbital overlap with one of the F2p orbitals. The other two F2p orbitals remain as non-bonding orbitals. MO from d Orbitals. Start from the third row, all the elements after sodium (Na) have d .
Gods Unchained represents one of the popular options if you want to play free online games to earn money. This is an online trading card game you can play for free and earn virtual currency. The .
iodine orbital diagram|Iodine orbital diagram